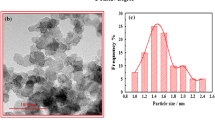
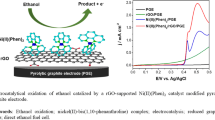
Abstract
Nickel modified graphite electrodes (G/Ni) prepared by galvanostatic deposition were examined for their redox process and electrocatalytic activities towards the oxidation of methanol, ethanol, 1-propanol and 2-propanol in alkaline solutions. The methods of cyclic voltammetry (CV), chronoamperometry (CA) and impedance spectroscopy (EIS) were employed. In CV studies, the electrochemical response, peak current varied in the order of MeOH > EtOH > 1-PrOH > 2-PrOH. Under the CA regime, a higher catalytic rate constant obtained for methanol oxidation was in agreement with CV measurements. Lower charge transfer resistance was obtained for low carbon alcohols oxidation and significantly higher exchange current density was obtained for methanol oxidation.
Similar content being viewed by others
References
Danaee I, Jafarian M, Gobal F, Sharafi M, Mahjani MG. Electrochemical impedance of ethanol oxidation in alkaline eedia. Chem Res Chinese Universities, 2012, 28: 19–25
Vigier F, Coutanceau C, Hahn F, Belgsir EM, Lamy C. On the mechanism of ethanol electro-oxidation on Pt and PtSn catalysts: Electrochemical and in situ IR reflectance spectroscopy studies. J Electroanal Chem, 2004, 563: 81–89
Secanell M, Wishart J, Dobson P. Computational design and optimization of fuel cells and fuel cell systems: A review. J Power sources, 2011, 196: 3690–3704
Feng L, Zhang J, Cai W, Liang L, Xing W, Liu C. Single passive direct methanol fuel cell supplied with pure methanol. J Power sources, 2011, 196: 2750–2753
Danaee I, Jafarian M, Mirzapoor A, Gobal F, Mahjani MG. Electrooxidation of methanol on NiMn alloy modified graphite electrode. Electrochim Acta, 2010, 55: 2093–2100
Feng L, Zhao X, Yang J, Xing W, Liu C. Electrocatalytic activity of Pt/C catalysts for methanol electrooxidation promoted by molybdovanadophosphoric acid. Catal Commun, 2011, 14: 10–14
Lamy C, Lima A, LeRhun V, Delime F, Coutanceau C, Léger JM. Recent advances in the development of direct alcohol fuel cells (DAFC). J Power sources, 2002, 105:283–296
Rodrigues LDA, De Souza JPI, Pastor E, Nart FC. Cleavage of the C-C bond during the electrooxidation of 1-propanol and 2-propanol: Effect of the Pt morphology and of codeposited Ru. Langmuir, 1997, 13: 6829–6835
Sun SG, Lin Y. In situ FTIR spectroscopic investigations of reaction mechanism of isopropanol oxidation on platinum single crystal electrodes. Electrochim Acta, 1996, 41: 693–700
Fujiwara N, Siroma Z, Ioroi T, Yasuda K. Rapid evaluation of the electrooxidation of fuel compounds with a multiple-electrode setup for direct polymer electrolyte fuel cells. J Power Sources, 2007, 164: 457–463
Liu J, Ye J, Xu C, Jiang SP, Tong Y. Electro-oxidation of methanol, 1-propanol and 2-propanol on Pt and Pd in alkaline medium. J Power Sources, 2008, 177: 67–70
Santasalo A, Vidal-Iglesias FJ, Solla-Gullón J, Berná A, Kallio T, Feliu JM. Electrooxidation of methanol and 2-propanol mixtures at platinum single crystal electrodes. Electrochim Acta, 2009, 54: 6576–6583
Cheng Y, Liu Y, Cao D, Wang G, Gao Y. Effects of acetone on electrooxidation of 2-propanol in alkaline medium on the Pd/Ni-foam electrode. J Power Sources, 2011, 196: 3124–3128
Danaee I, Jafarian M, Forouzandeh F, Gobal F, Mahjani MG. Electrocatalytic oxidation of methanol on Ni and NiCu alloy modified glassy carbon electrode. Int J Hydrogen Energy, 2008, 33: 4367–4376
Antolini E. Catalysts for direct ethanol fuel cells. J Power sources, 2007, 170: 1–12
Danaee I, Jafarian M, Forouzandeh F, Gobal F, Mahjani MG. Electrochemical impedance studies of methanol oxidation on GC/Ni and GC/NiCu electrode. Int J Hydrogen Energy 2009, 34: 859–869
Tian T, Liu C, Liao J, Xing W, Lu T. The enhancement effect of Eu3+ on electro-oxidation of ethanol at Pt electrode. J Power sources, 2007, 174: 176–179
Antolini E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater Chem Phys 2003, 78: 563–573
King WD, Corn JD, Murphy OJ, Boxall DL, Kenik EA, Kwiatkowski KC. Pt-Ru and Pt-Ru-P/carbon nanocomposites: Synthesis, characterization, and unexpected performance as direct methanol fuel cell (DMFC) anode catalysts. J Phys Chem, 2003,107: 5467–5474
Park IS, Park KW, Choi JH, Park CR, Sung YE. Electrocatalytic enhancement of methanol oxidation by graphite nanofibers with a high loading of PtRu alloy nanoparticles. Carbon, 2007, 45: 28–33
Feng L, Yan L, Cui Z, Liu C, Xing W. High activity of Pd-WO3/C catalyst as anodic catalyst for direct formic acid fuel cell. J Power sources, 2011, 196: 2469–2474
Cui Z, Feng L, Liu C, Xing W. Pt nanoparticles supported on WO3/C hybrid materials and their electrocatalytic activity for methanol electro-oxidation. J Power sources, 2011, 196: 2621–2626
Wen TC, Lin SM, Tsai JM. Sulphur content and the hydrogen evolving activity of NiSx deposits using statistical experimental strategies. J Appl Electrochem, 1994, 24: 233–238
Fan C, Piron DL, Sleb A, Paradis P. Study of electrodeposited nickel-molybdenum, nickel-tungsten, cobalt-molybdenum, and cobalt-tungsten as hydrogen electrodes in alkaline water electrolysis. J Electrochem Soc, 1994, 141: 382–387
Raj LA, Vasu KI. Transition metal-based hydrogen electrodes in alkaline solution—electrocatalysis on nickel based binary alloy coatings. J Appl Electrochem, 1991, 32: 32–38
Casadei MA, Pletcher D. The influence of conditions on the electrocatalytic hydrogenation of organic molecules. Electrochim Acta, 1988, 33: 117–1120
Berchmans S, Gomathi H, Prabhakara Rao G. Electrooxidation of alcohols and sugars catalysed on a nickel oxide modified glassy carbon electrode. J Electroanal Chem, 1995, 394: 267–270
Fleischmann M, Korinek K, Pletcher D. The kinetics and mechanism of the oxidation of amines and alcohols at oxidecovered nickel, silver, copper, and cobalt electrodes. J Chem Soc Perkin Trans, 1972, 2: 1396–1402
Taraszewska J, Roslonek G. Electrocatalytic oxidation of methanol on a glassy carbon electrode modified by nickel hydroxide formed by ex situ chemical precipitation. J Electroanal Chem, 1994, 364: 209–213
Danaee I. Kinetics and mechanism of palladium electrodepositon on graphite electrode by impedance and noise measurements. J Electroanal Chem, 2011, 662: 415–420
El-Shafei AA. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium. J Electroanal Chem, 1999, 471: 89–95
Jafarian M, Forouzandeh F, Danaee I, Gobal F, Mahjani MG. Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. J Solid State Electrochem, 2009, 13: 1171–1179
Hahn F, Beden B, Croissant MG, Lamy C. In situ UV visible reflectance spectroscopic investigation of the nickel electrodealkaline solution interface. Electrochim Acta, 1986, 31: 335–342
Desilvestro J, Corrigan DA, Weaver MJ. Characterization of redox states of nickel hydroxide filmelectrodes by in situ surface Raman spectroscopy. J Electrochem Soc, 1988, 135: 885–892
Barnard R, Randell CF. Studies concerning charged nickel hydroxide electrodes. VII. Influence of alkali concentration on anodic peak positions. J Appl Electrochem, 1983, 13: 89–95
Fleischmann M, Korinek MK, Pletcher D. The oxidation of organic compounds at a nickel anode in alkaline solution. J Electroanal Chem, 1971, 31: 39–49
Danaee I, Jafarian M, Forouzandeh F, Gobal F, Mahjani MG. Kinetic interpretation of a negative time constant impedance of glucose electrooxidation. J Phys Chem B, 2008, 112: 15933–15940
Danaee I, Jafarian M, Forouzandeh F, Gobal F, Mahjani MG. Impedance spectroscopy analysis of glucose electro-oxidation on Ni-modified glassy carbon electrode. Electrochim Acta, 2008, 53: 6602–6609
Bard AJ, Faulkner LR. Electrochemical Methods. New York: Wiley, 2001
Danaee I, Noori S. Kinetics of the hydrogen evolution reaction on NiMn graphite modified electrode. Int J Hydrogen Energy, 2011, 36: 12102–12111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafarian, M., Mirzapoor, A., Danaee, I. et al. A comparative study of the electrooxidation of C1 to C3 aliphatic alcohols on Ni modified graphite electrode. Sci. China Chem. 55, 1819–1824 (2012). https://doi.org/10.1007/s11426-012-4731-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4731-6